DESCRIPTION
ViroSafe™ MΤM (Molecular Transport Medium) is a reliable and safe medium that completely inactivates infectious agents, such as COVID-19, monkeypox and other viruses, as well as bacterial, fungal and mycobacterium tuberculosis pathogens.
ViroSafe™ MΤM is intended for the stabilization, transportation, and inactivation of infectious agents (viruses, bacteria, fungi) and is an approved transport medium for COVID-19 diagnostic swab samples.
ViroSafe™ MΤM protects and stabilizes the integrity of infectious agent DNA and RNA for subsequent extraction and analysis for diagnostic or research purposes.
ViroSafe™ MΤM inactivates the pathogens, therefore, providing a safe method for transporting clinical specimens and processing by laboratory technologists. The medium can be also used for long-term deep-freezing storage of collected clinical samples without significant loss in the integrity of their RNA or DNA.
Clinical samples in our ViroSafe™ medium tubes can be transported at room temperature, making them the ideal choice for long distance safe transportation. Our comparison study between transporting same viral samples at 2-8 ᴼC and room temperature (15-25 ᴼC) in ViroSafe™ medium tubes (up to 96 hours) showed insignificant difference in their RNA signals (RT-PCR).
Unlike other commercially available media, our experiments showed that clinical specimens in ViroSafe™ MΤM can stored at room temperature (15-27 ᴼC) for at least 2 months for RNA, 2 years for DNA, and minimum 6 moths at 4 ᴼC for RNA, and indefinitely at ˂ -20 ᴼC for RNA with minimal loss (≤ 10%) in their analyzed RNA signals using RT-PCR.
ViroSafe™ medium can be also used to store purified RNA and DNA from any source indefinitely in a freezer (-20 ᴼC). the Medium is also suitable for preserving, transportation and storage of biopsy or small tissue samples for RNA extraction purposes.
ViroSafe™ medium is universally compatible with existing nucleic acid extraction workflows (including those used for COVID19 RNA extraction), specifically on leading automated platforms for high-throughput sample processing. Platforms include Roche MagnaPure, Qiagen (both Manual Extraction Mini kits and QiaSymphony), Cepheid GeneXpert, BioMerieux NucliSENS easyMAG, Luminex, Maglead, GE Life Sciences Cytiva System, and Versant.
ViroSafe™ MΤM is compatible with COVID-19 testing using the following RT-PCR platforms: Roche (Cobas, MagnaPure, and Lightcycler), Thermo Fisher (Applied Biosystems ABI 7500, Kingfisher MagMax, and QuantStudio), and Abbott M2000.
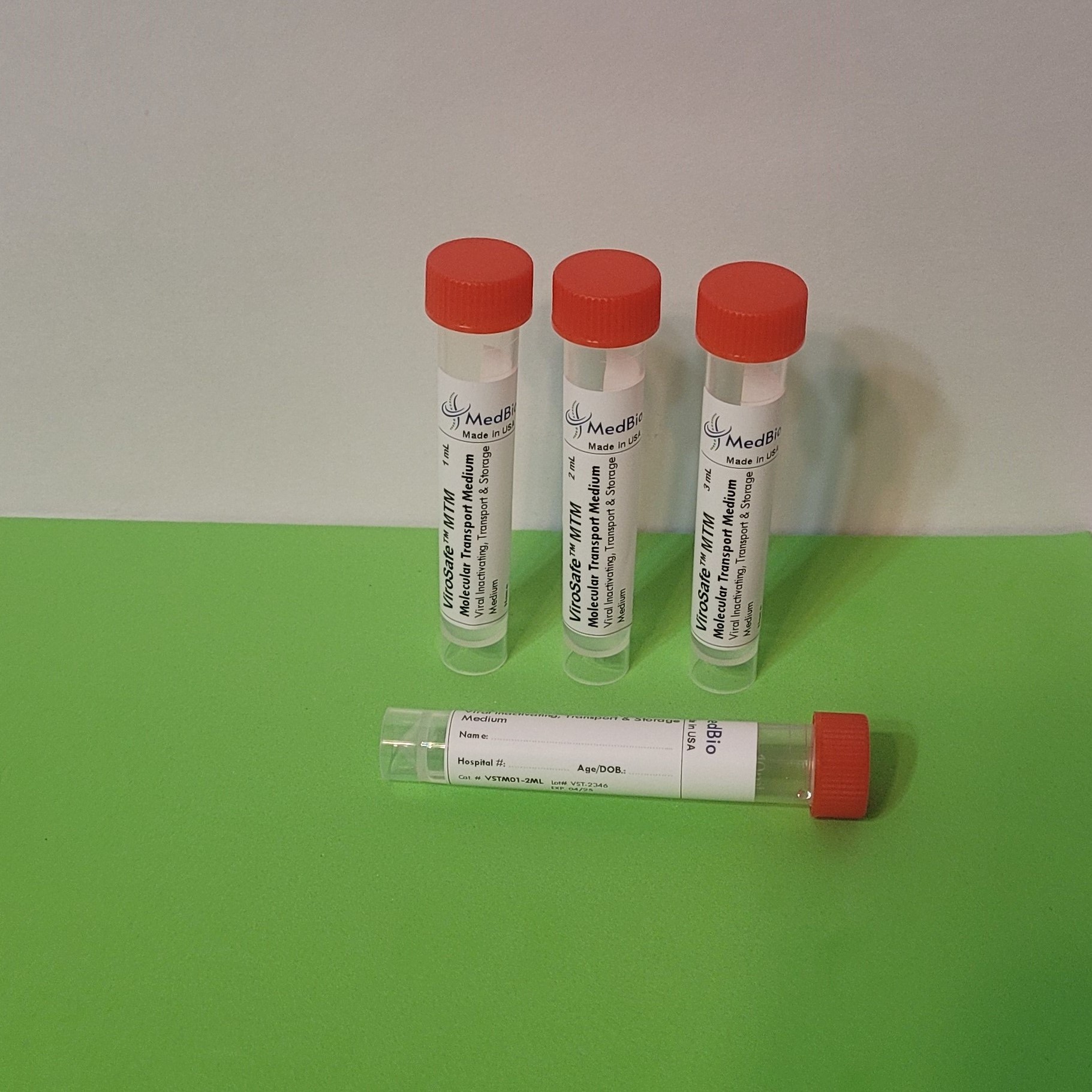
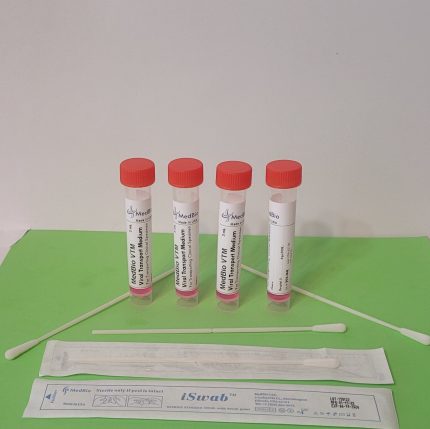
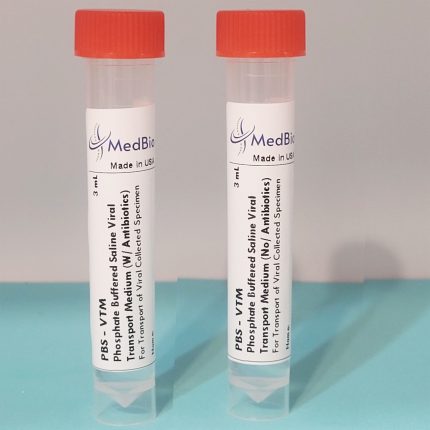
ViroSafe™ MΤM is provided as Ready-To-Use Sampling Medium in pre-labeled transparent polypropylene screwcap tubes/vials containing 1 mL, 2 mL, and 3 mL volumes. Economic Bulk medium is also available in 250 mL and 500 mL screwcap cell culture-grade transparent PET/PETG bottles, allowing laboratories to aliquot their own tubes/vials.
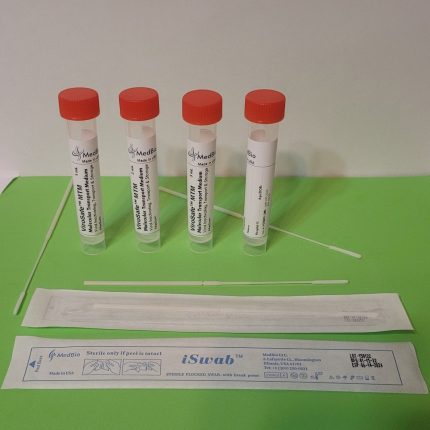
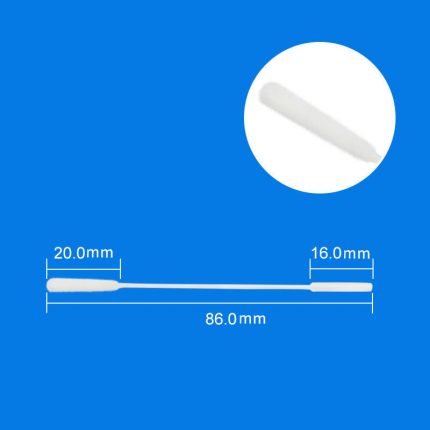
ViroSafe™ MΤM is also available in a kit format containing one viroSafe™ medium tube (1mL, 2 mL, or 3 mL), one swab (Nasal/Nasopharyngeal iSwab™, oral/oropharyngeal iSwab™, or general sample collection iSwab™), and one biohazard specimen Ziplock bag. Please see products under ViroSafe™ MΤM Nasal Sampling & Transport Kits & ViroSafe™ MΤM Oral Sampling & Transport Kits.
Our tubes/vials have the following criteria: Screwcap, Upright free-standing tube with Conical bottom, Clear engraved graduation (0.5 to 10 mL), Sterile, DNase/RNase-free, Cryogenic, Leak-proof, Shatter-proof and can be Centrifuged. Tube internal dimensions: 94 x Ø14.8 mm, and external dimensions: 95 x Ø 15.9 mm. Tube volume is 10 mL. We can accommodate packaging the medium in any tube size of your choice!
Our medium is produced under highest manufacturing standards. It is formulated from highly pure molecular-grade chemicals in Autoclaved 18.2 mega Ω-cm DNase-RNase-free deionized water.
The medium is sterilized by filtration using 1 µm then 0.22 µM filter cartridges.
Each batch of ViroSafe™ MΤM product is extensively evaluated for efficacy, stability, sterility and confirmed for absence of any DNase/RNase and protease activity.
COMPOSITION:
ViroSafe™ MΤM is developed in our research laboratory at Med Biosciences and modified to enhance performance and stability. The medium is composed of 3.0 M Sodium Thiocyanate, Protein Solubilizing Agent, Buffering Agent (pH 7.6 ± 0.1), chelating agent, Salts, Organic Alcohol, and Reducing Agent.
PACKAGING:
100 ViroSafe™ MΤM tubes (1 mL, 2 mL, or 3 mL each) are racked in one carton box.
4, 8, and 12 Boxes (each contains 100 tube) are packaged in one large carton (400-1200 tubes).
Minimum order: 100 tubes (1 Box).
We accommodate customized packaging requests (tube size, liquid volume, carton size, labeling, …etc.). Please get in touch with our team for your requests of customized packaging.
STORAGE TEMP
2-25 ᴼC
SHELF LIFE
Minimum 1 year at room temperature.
2 years at 2-8 ᴼC
NOTES
Med Biosciences is authorized by FDA to Manufacture and market viral transport media.
Each batch of ViroSafe™ medium is vigorously evaluated for efficacy, sterility, and stability. The transport medium is sterile filtered (using 0.22 µm filter), and RNase and DNase free.
Product is stable and shipped at room temperature.
ViroSafe™ MΤM is incompatible with Hologic RT-PCR platforms, when post assay bleach disinfectant is used.
This transport medium contains guanidine thiocyanate. When exposed to bleach (sodium hypochlorite),
a chemical reaction occurs releasing cyanide gas.
Please contact us if you have special shipping or packaging requirement. We can accommodate most requests!
Request a discount price offer for orders ≥ 2400 tubes.












Reviews
There are no reviews yet.