DESCRIPTION
Med Biosciences VΤM Viral Transport Medium provides an appropriate transport and storage environment for clinical sampled swabs. Our VΤM is intended for transporting clinical specimens suspected to contain SARS-CoV-2 (COVID-19) for subsequent testing using biochemical, molecular assays (RNA extraction and PCR/RT-PCR), antigen assays, or other validated diagnostic protocols.
The Medium is formulated following the United States Center for Disease Control CDC SOP# DSR-052-05, Preparation of Viral Transport Medium under FDA device listing D411652 (Code JSG, Exempt).
Med Biosciences (MedBio, LLC.) is authorized by FDA to commercially manufacture and distribute Viral Transport Medium VΤM as set forth in Section IV.B of the FDA’s COVID-19 Transport Media Policy.
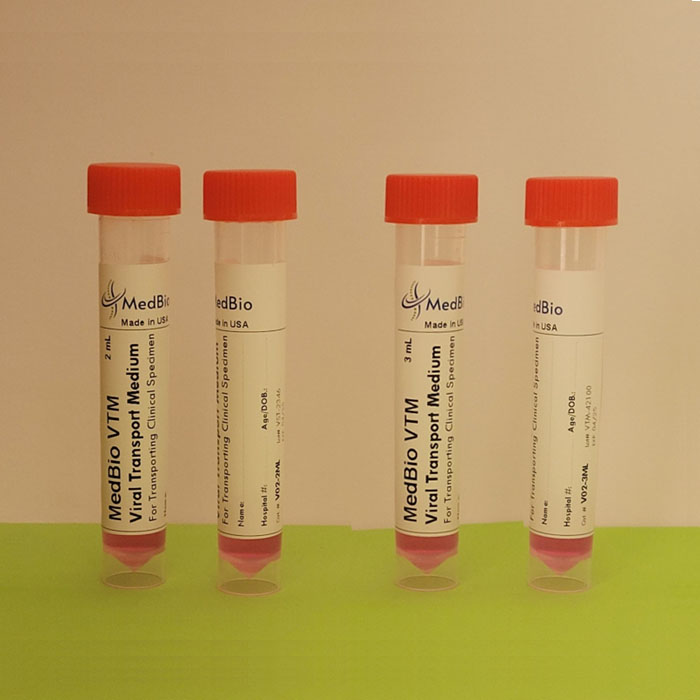
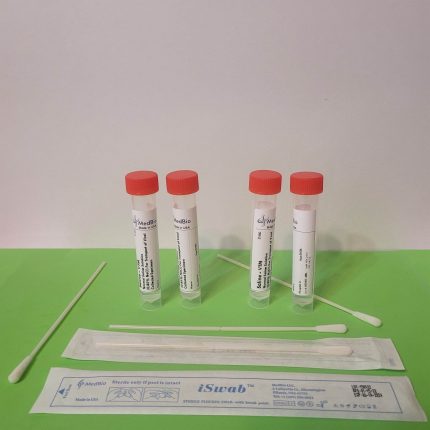
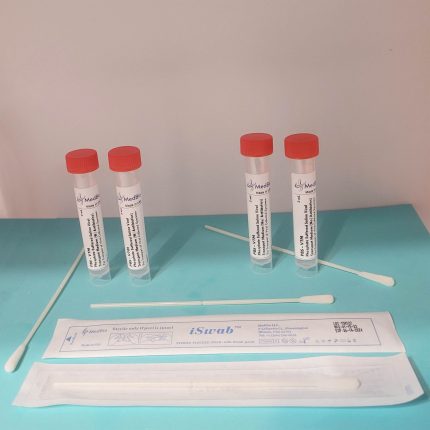
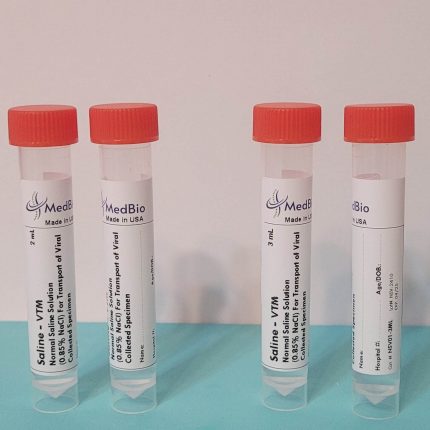
Our VΤM is provided as Ready-To-Use Sample Transporting Medium in pre-labeled transparent polypropylene screwcap tubes containing 2 mL and 3 mL volumes. Economic Bulk medium is also available in 250 mL-1000 mL screwcap cell culture-grade transparent PET/PETG bottles, allowing laboratories to aliquot their own tubes/vials. We also offer ready bulk medium up to 50 L, and blended powder medium kits for making 25 L and 50 media.
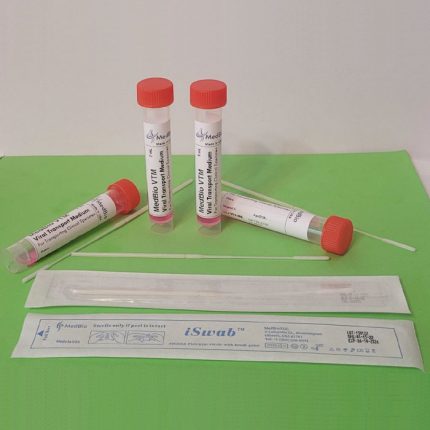
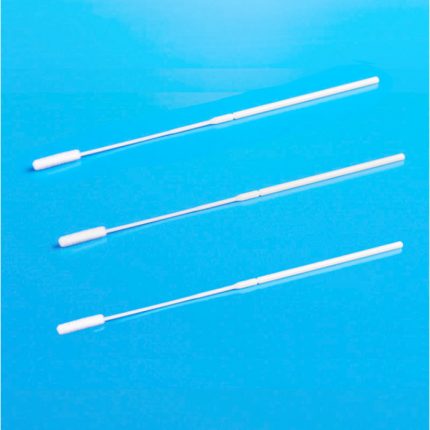
Our VΤM is also available in a kit format containing one medium tube (2 mL, or 3 mL), one swab (Nasal/Nasopharyngeal iSwab™, oral/oropharyngeal iSwab™, or general sample collection iSwab™), and one biohazard specimen Ziplock bag. Please see products under VΤM Nasal Sampling & Transport Kits & Oral Sampling & Transport Kits.
Our tubes have the following criteria: Screwcap, Upright free-standing tube with Conical bottom, Clear engraved graduation (0.5 to 10 mL), Sterile, DNase/RNase-free, Cryogenic, Leak-proof, Shatter-proof, and can be centrifuged. Tube internal dimensions: 94 x Ø14.8 mm, and external dimensions: 95 x Ø 15.9 mm. Tube volume is 10 mL. We can accommodate packaging the medium in any tube size of your choice!
Our medium is produced under the highest manufacturing standards. It is formulated from highly pure molecular-grade chemicals in Autoclaved 18.2 mega Ω-cm DNase-RNase-free deionized water.
The high-quality heat-inactivated fetal bovine serum (FBS) used in our medium formulation is obtained from a USA dependable source and extensively tested for mycoplasma and other pathogens.
The medium is sterilized by filtration using 0.22 µM filter cartridges.
Each batch of our VΤM products is extensively evaluated for efficacy, stability, and sterility, and confirmed for the absence of any DNase/RNase and protease activity.
COMPOSITION:
The medium is composed of 1X Hanks Balanced Salt Solution (HBSS), 2% Heat Inactivated FBS (Made in the USA), 50 mg/mL Gentamicin Sulfate, 250 µg/mL Amphotericin B, and 0.0003% Phenol Red (pH 7.3 ± 0.1).
PACKAGING:
100 VΤM Tubes (2 mL or 3 mL each) are racked in one box.
4, 6, 8, and 12 boxes (100 tubes each) are packaged in one large carton (400 to 1200 tubes).
Minimum order: 100 tubes.
We accommodate customized packaging requests. Please contact us for your request for customized packaging.
STORAGE TEMP
2-8 ᴼC
SHELF LIFE
2 years at 2-8 ᴼC
NOTES
Med Biosciences is authorized by FDA to Manufacture and distribute VΤM. The authorization can be accessed using the following link: https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/faqs-viral-transport-media-during-covid-19
Each batch of our VΤM is vigorously evaluated for efficacy, sterility, and stability. The transport medium is sterile filtered (using 0.22 µm filter), and RNase and DNase free.
The product is stable and shipped at room temperature. Store at 2-8 ᴼC upon receiving for extended shelf life.
Please contact us if you have special shipping or packaging requirements. We can accommodate most requests!
Request a discount price offer for orders ≥ 2400 tubes.












Reviews
There are no reviews yet.